- January 05, 2015
- Taiho Pharmaceutical Co., Ltd.
Taiho Pharmaceutical Receives Approval to Manufacture and Market ZOSYN® 4.5 for I.V. Infusion Bag, an Injectable Antibiotic Combined with β-Lactamase Inhibitor
Taiho Pharmaceutical Co., Ltd. (HQ: Tokyo, President and Representative Director: Masayuki Kobayashi) announced that it received approval on December 26, 2014 to manufacture and market ZOSYN® 4.5 for I.V. infusion bag, an injectable antibiotic combined with β-lactamase inhibitor (generic name: tazobactam and piperacillin) as a new formulation of ZOSYN®.
ZOSYN® 4.5 for I.V. infusion bag is a double-bag containing tazobactam and piperacillin in a ratio of 1:8 (same as ZOSYN® 4.5 vial) in its upper compartment and saline solution 100 mL in its lower compartment. In addition to making it easier for medical facilities to dissolve the drug in solution, the I.V. infusion bag also prevents bacterial and other types of contamination, prevents medical errors during the preparation of the drug, and facilitates prompt use during emergency procedures.
Taiho Pharmaceutical remains committed to making further contribution to patients and medical professionals engaged in the treatment of infectious diseases.
About ZOSYN®
ZOSYN® is an injectable antibacterial combination product consisting of the β-lactamase inhibitor tazobactam that Taiho discovered and the semisynthetic antibiotic piperacillin that Toyama Chemical Co., Ltd. (HQ: Tokyo, President and Representative Director: Masuji Sugata) discovered, in a ratio of 1:8 in a potency for intravenous administration.
Taiho holds the manufacturing and marketing authorization for ZOSYN® in Japan, and Taisho Toyama Pharmaceutical Co., Ltd. (HQ: Tokyo, President and Representative Director: Akira Ohira) is marketing the product. In July 2008, ZOSYN® was approved in Japan for the treatment of septicemia, pneumonia, pyelonephritis, and complicated cystitis in adults and children. In September 2012, it received approval for the indications of peritonitis, intra-abdominal abscess, cholecystitis, and cholangitis. ZOSYN® is approved in 102 countries.* ZOSYN® is recommended in the guidelines issued by medical associations in the U.S., Europe, and elsewhere for treatment of various infectious diseases, and is well recognized as a standard therapeutic agent for bacterial infections around the world.
*As of October 2012 (latest data)
Product Information
Product Name
ZOSYN® 4.5 for I.V. infusion bag
Indications
Indicated bacteria:
Susceptible strains of Staphylococcus spp., Streptococcus spp., Streptococccus pneumoniae,Enterococcus spp., Moraxella (Branhamella) catarrhalis, Escherichia coli, Citrobacter spp., Klebsiellaspp., Enterobacter spp., Serratia spp., Proteus spp., Providencia spp., Haemophilus influenzae,Pseudomonas aeruginosa, Acinetobacter spp., Peptostreptococcus spp., Clostridium spp. (except C.difficile), Bacteroides spp., Prevotella spp.
Indications:
Septicemia, pneumonia, pyelonephritis, complicated cystitis, peritonitis, intra-abdominal abscess, cholecystitis, and cholangitis
Dosage and administration
For septicemia, pneumonia, peritonitis, intra-abdominal abscess, cholecystitis, and cholangitis:
The usual dose of ZOSYN® for I.V. infusion bag for adults is a single 4.5 g dose of tazobactam and piperacillin, administered intravenously three times daily. In the case of pneumonia, the dosage may be increased to four times daily according to the symptoms and clinical condition. The usual dose forchildren is a 112.5 mg/kg of body weight per single dose, administered intravenously three times daily. The single-dose amount may be decreased according to the symptoms and clinical condition.The upper limit of a single dose for children is equal to the adult dosage of 4.5 g of tazobactam and piperacillin.
For pyelonephritis and complicated cystitis:
The usual dose of ZOSYN® for I.V. infusion bag for adults is a single 4.5 g dose of tazobactam and piperacillin, administered intravenously twice daily. The dosage may be increased to three times daily according to the symptoms and clinical condition. The usual dose for children is a 112.5 mg/kgof body weight per single dose, administered intravenously twice daily. The dosage may be decreased according to the symptoms and clinical condition. The dosage may also be increased to three times per day in accordance with the symptoms and the clinical condition. However, the upper limit of a single dose for children is equal to the adult dosage of 4.5g of tazobactam and piperacillin.
Instructions for dissolving the drug in solution:
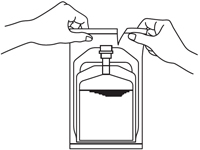
1. Remove the outer wrapper immediately before use.
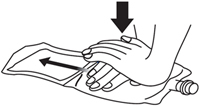
2. Press down on the solution compartment with the hands until the barrier is broken. Make sure the drug is completely dissolved in the solution.
Alternately pressing down on the drug compartment and the solution compartment makes it easier to dissolve the drug in the solution.
3. Make sure the drug is dissolved.
After performing the above procedure, remove the anti-contamination sheet on the rubber stopper and place the bag in the infusion set. Use immediately after dissolving the drug.
Package
ZOSYN® 4.5 for I.V. infusion bag: 10 kits
Information in this news release was current as of the original release date.
Taiho Pharmaceutical's news releases are intended to provide information to the media. It may contain information about ethical drugs or products under development, however information contained in the news releases are not intended to constitute promotion, advertisement, or medical advice.