Globalization
Taiho Pharmaceutical’s Future
Taiho Pharmaceutical is expanding its global reach, looking to become a specialty pharma trusted worldwide. The company conducts drug discovery R&D in collaboration with group companies in the U.S., Europe, Asia and Oceania. It is building its own marketing structures and expanding its sales network. In recent years, the company has established two corporate venture capital firms in order to expand its development pipeline and create innovation. Taiho Pharmaceutical aspires to make even greater contributions to medical care worldwide.
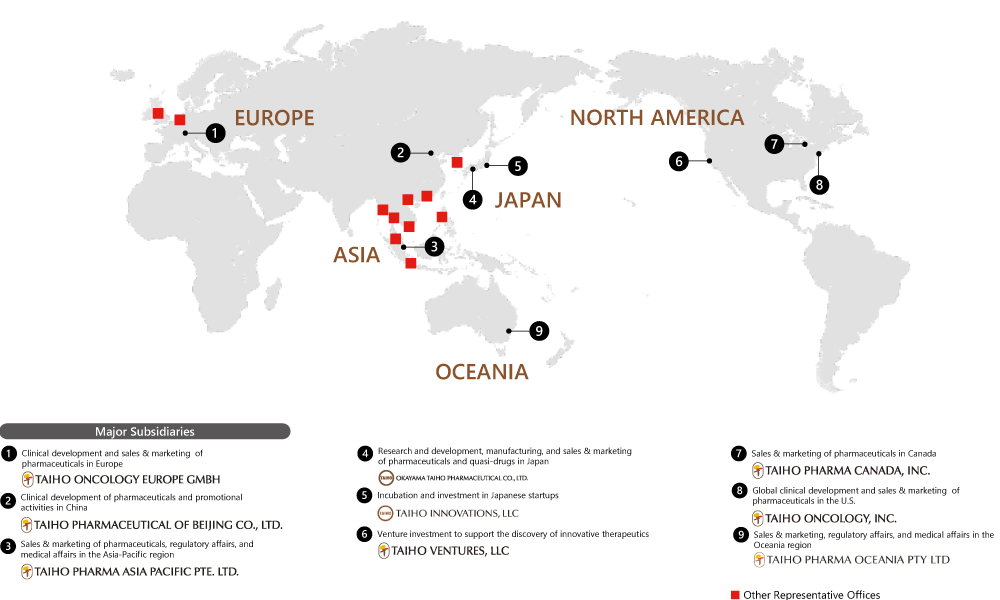
Message from EUROPE
Taiho group’s global expansion: advancing cancer care in Europe
Peter Foertig
General Manager
TAIHO ONCOLOGY EUROPE GMBH
Taiho Oncology Europe, established in 2021, is dedicated to delivering innovative cancer treatments across Europe. As part of the Taiho Pharmaceutical group, we are building a strong medical and sales organisation and are now marketing futibatinib ourselves in several European countries. Backed by state-of-the-art research labs in Japan, we continue to expand our oncology portfolio, ensuring the proper use of our therapies. Through global collaboration and cutting-edge science, we remain committed to shaping the future of cancer care—bringing hope, health, and smiles to patients, their families, and caregivers worldwide.
Message from ASIA
Aiming to deliver innovative new drugs to as many people in Asia and Oceania as possible
Yoshimasa Nishimura
Managing Director
TAIHO PHARMA ASIA PACIFIC PTE. LTD.
We uphold the shared slogan, “By Local. For Local” while also playing a key role in Taiho Pharmaceutical’s global initiatives. Our primary mission is to provide the innovative new drugs and unique consumer healthcare products developed by Taiho Pharmaceutical to medical professionals, to patients, and consumers across countries and regions in Asia and Oceania, through the caring hands of our local employees. Our entire team is united to give our best effort every day to bring smiles to the faces of as many locals as possible.
Message from
NORTH AMERICA
Expanding our impact in oncology worldwide
Timothy Whitten
President & CEO
TAIHO ONCOLOGY, INC.
At Taiho Oncology, we are committed to improving the lives of patients with cancer, their families and their caregivers by developing and commercializing anti-cancer agents for various tumor types to treat solid tumors and hematological malignancies.
Since 2002, our scientific diligence and acute focus in oncology have enabled us to establish a world-class record of success with orally administered therapies in multiple oncological disease states and across geographic borders in concert with Taiho Japan headquarters.
Headquartered in Princeton, New Jersey, U.S., Taiho Oncology is responsible for global development and North American and European commercialization of a portfolio of anticancer drugs for an array of solid tumors and hematological malignancies.




