Globalization
Taiho Pharmaceutical’s Future
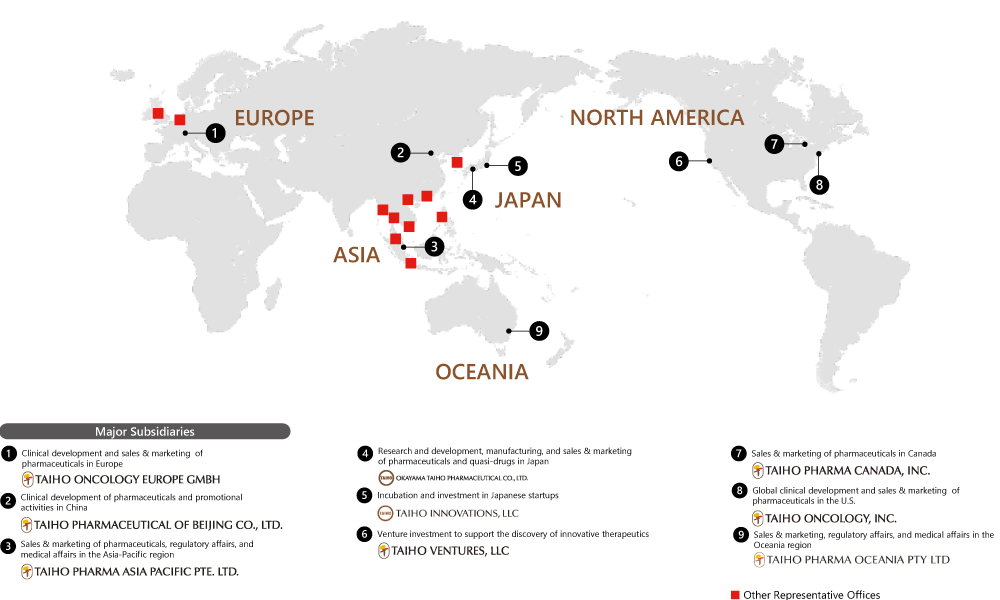
Taiho Pharmaceutical is expanding its global reach, looking to become a specialty pharma trusted worldwide. The company conducts drug discovery R&D in collaboration with group companies in the U.S., Europe, Asia and Oceania. It is building its own marketing structures and expanding its sales network. In recent years, the company has established two corporate venture capital firms in order to expand its development pipeline and create innovation. Taiho Pharmaceutical aspires to make even greater contributions to medical care worldwide.
Message from EUROPE
Properly delivering Taiho Pharmaceutical’s new drugs to patients in Europe
Atsushi Azuma, Ph.D.
Chairman
TAIHO ONCOLOGY EUROPE GMBH
Established in January 2021 as a marketing company in Europe for the Taiho group, Taiho Oncology Europe is collaborating with other Taiho group companies to build its own sales/ marketing organization for the launch of the novel oral anti-cancer agent futibatinib (development code: TAS-120), an FGFR inhibitor currently under development. Going forward, we hope to contribute to health and smiles for patients in Europe by promoting the proper use of our drugs and further expanding our lineup of anticancer drugs while strengthening our organization.
Message from ASIA
Aiming to deliver innovative new drugs to as many people in Asia as possible
Yoshimasa Nishimura
Managing Director
TAIHO PHARMA ASIA PACIFIC PTE. LTD.
Taiho Pharmaceutical of Beijing and Taiho Pharma Asia Pacific share the slogan, “By Local. For Local.” Our primary mission is to provide the innovative new drugs and unique consumer healthcare products developed by Taiho Pharmaceutical to medical professionals, to patients, and to all the people of Asian countries and regions, through the caring hands of our local employees. Our entire team is united to give our best effort every day to bring smiles to the faces of as many locals as possible.
Message from
NORTH AMERICA
Expanding our sales network from a global perspective and bringing Taiho Pharmaceutical’s philosophy to the world
Timothy Whitten
President & CEO
TAIHO ONCOLOGY, INC.
Taiho Oncology has embraced the Taiho Pharmaceutical corporate philosophy and it guides us in every important decision we make. It helps us understand what we should do as we strive to improve human health and contribute to a society enriched by smiles. The corporate philosophy tells us we should expand commercially into other geographic markets, we should embrace globalization, and we should invest in our pipeline and marketed products
globally. By doing that, it will allow more patients to benefit from our products, provide more financial resources to invest in our pipeline and marketed products development, and help the Taiho name become more well-known globally.




