Evolving and developing proprietary platform technologies to deliver next-generation drug discovery

Evolving and developing proprietary platform technologies to deliver next-generation drug discovery
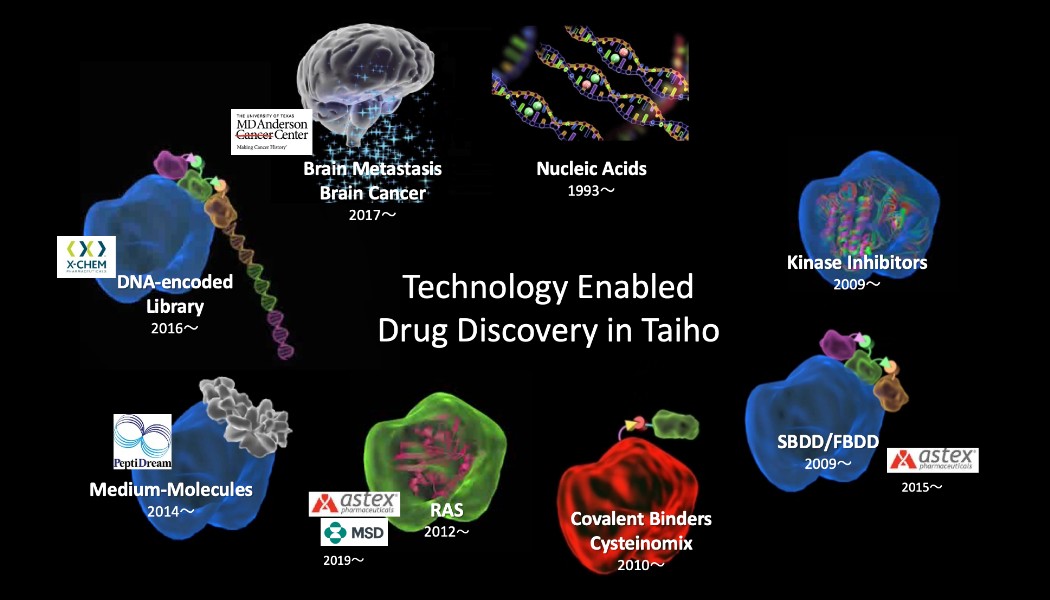
Taiho Pharmaceutical has dedicated our efforts to establish drug discovery platforms to ensure patients’ invaluable “Today and Every Day.” Taiho drug discovery started with the discovery of antimetabolites as oral anticancer agents, which opened the door to a diverse pipeline of compounds, and many of these compounds have advanced to the clinical stage. Below, we present an overview of the transition and current evolution of our drug-discovery platforms in the field of oncology.
Drug Discovery Research at Taiho Pharmaceutical
Evolution of Drug-Discovery Platforms
Open Innovation

In 1969, we began full-scale R&D in the field of oncology and not much later succeeded in launching several new drugs in Japan based on the strength of our discovery technology in antimetabolites that suppress the growth of cancer cells by inhibiting the formation of substances necessary for nucleic acid synthesis in cancer cells. LONSURF®, launched in Japan in 2014 as a treatment for colorectal cancer, is now available worldwide, having been approved in more than 100 countries and regions.
Based on the expertise in antimetabolite research we had accumulated over many years , our drug discovery research evolved to kinase drug discovery, which seeks to inhibit the kinases (phosphorylating enzymes) necessary for signal transduction when cancer cells proliferate. In the 2010s, we established a foundation for fragment-based (small molecule compounds) drug discovery. Since 2012, we have been developing mutant KRAS inhibitors. RAS drug discovery is said to be a difficult area for new drug discovery, but we have made significant progress by applying our proprietary drug-discovery platform technologies. (Learn more about RAS below)
Cysteinomix serves as the anchor for these proprietary drug-discovery platform technologies, forming the backbone of Taiho Pharmaceutical’s drug discovery. An FGFR inhibitor discovered using this technology was approved in the United States (accelerated approval) in 2022, in Japan in 2023, and in Europe (conditional marketing authorization).
Using Cysteinomix, Taiho is dedicated to creating a pipeline of new targets and focusing on enhancing our technology, particularly including the expansion of our compound library. To this end, we are accelerating our move to data-driven drug discovery using AI and robotics technologies. In one challenge we are embracing in the new modality area, we formed an alliance with PeptiDream Inc. in 2020 related to its proprietary Peptide Discovery Platform System (PDPS) technology. In addition to the natural product-derived library that we have accumulated over the years, we are expanding into medium-molecule drug discovery. Strategic drug discovery efforts that leverage the advantages of each are now beginning.
Kinase drug discovery: Enzymes responsible for the phosphorylation of proteins are called kinases, and abnormalities in kinases are said to cause the loss of cellular homeostasis and contribute to the proliferation of cancer cells. Kinase drug discovery involves targeting these molecules with inhibitors to suppress cell proliferation and metastasis.
Fragment-based drug discovery: A method of developing bioactive substances we like to think of as “ unlocking the impossible.” It involves screening small molecules with a molecular weight of less than 300, known as fragments, to search for molecules that efficiently bind to target proteins.
RAS is a family of proteins involved in cell proliferation and other functions. There are three types: KRAS, NRAS, and HRAS. It is believed that when a mutation occurs in the genes for any one of these three types, abnormal RAS proteins are produced, causing cells to continue to proliferate, increasing the likelihood of cancer development. Among these, KRAS is one of the most frequently mutated oncogenes in human cancers. It is known that mutations in the KRAS gene are often detected in cancers with particularly high mortality rates, such as pancreatic cancer, colorectal cancer, and lung cancer. More than 30 years have passed since the discovery that mutations in the RAS gene are driver mutations in cancer, but it is still an extremely challenging target area for drug discovery. Taiho Pharmaceutical has paved the way for drug discovery targeting the KRAS gene by applying its proprietary Cysteinomix technology. We have been working on KRAS project since 2012 and have successfully identified KRAS G12C inhibitors with covalent binding mechanism .
In December 2019, Taiho Pharmaceutical entered into an exclusive agreement for global research collaboration and licensing, focused on small molecule inhibitors in development against multiple drug targets including the KRAS oncogene, with Astex Pharmaceuticals, a subsidiary of Otsuka Pharmaceutical, and Merck & Co., Inc., of Kenilworth, NJ, USA (MSD). Through a strategic collaboration in 2021, the focus was expanded to other targets related to KRAS, such as SHP2, and under the partnership, efforts are being made to bring good drugs to the world.
Drug Discovery Research at Taiho Pharmaceutical
Evolution of Drug-Discovery Platforms
Open Innovation