History of Pharmaceutical Business (Anticancer Drug Development)
Leveraging extensive evidence and a wealth of experience gained over half a century of anticancer drug development, Taiho Pharmaceutical will continue to develop new drugs that contribute to cancer patients around the world.
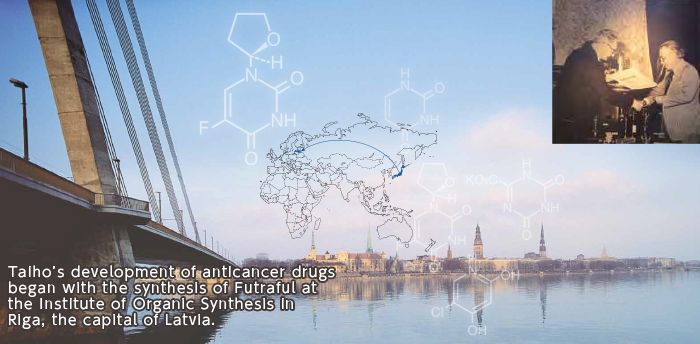
Encountering Futraful: The Beginning of Our Anticancer Drug Development
In 1969, Taiho Pharmaceutical’s first president, Yukio Kobayashi, visited the former Soviet Union for business discussions. While there, he encountered Futraful, a derivative* of a novel chemical compound, 5-fluorouracil (5-FU). Kobayashi sensed its great potential. He instantly decided to conduct clinical trials in Japan, and the company succeeded in development after overcoming many hurdles. In 1974, the oral anticancer drug Futraful, which enabled oral administration, was developed together with an injectable. Oral Futraful enabled outpatient therapy, which later led to the establishment of the concept of adjuvant chemotherapy.
*Derivative: A compound created by a change in part of a molecule of a given compound.
1974
Futraful capsule 200mg and Futraful injection 400mg launched in Japan
-
-

- The photo shows products at the time of initial launch.
-
Pursuing the Advancement of Anticancer Drugs: Overcoming Various Challenges
Pursuing the efficiency of Futraful, further research led to the launch of combination drugs in Japan: UFT in 1984 and TS-1 in 1999.
1984
UFT combination capsule T100 launched in Japan
-
-
- The photo shows products at the time of initial launch.
-
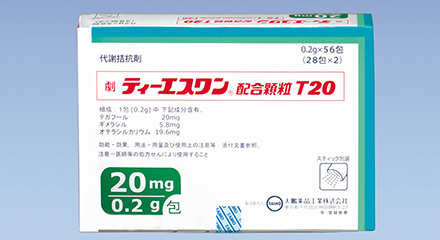
1999
TS-1 combination capsule T20/T25 launched in Japan
-
-

- The photo shows products at the time of initial launch.
-
2003
UZEL tablet 25mg launched in Japan
-
-
- The photo shows the half-weight type.
-
2009
TS-1 combination granule T20/T25 launched in Japan
Pioneering the Future of Cancer Treatment: Continuing to Improve Patients’ Quality of Life
In 2013, Taiho Pharmaceutical launched TS-1 as the world’s first anticancer agent in orally disintegrating (OD) tablet form. This helps to improve the quality of life of patients since it can be taken with or without water. In recent years, Taiho Pharmaceutical has been conducting R&D focused on comprehensive care in oncology, including cancer supportive care.
2010
Aloxi I.V. injection 0.75mg launched in Japan
Launched Abraxane I.V. infusion 100mg in Japan.
2013
TS-1 combination OD tablet T20/T25 and E-fen buccal tablet launched in Japan
-
-

- The photo shows products at the time of initial launch.
-
2015
Yondelis I.V. infusion 0.25mg/1mg launched in Japan
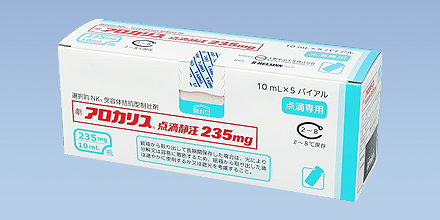
2022
Arokaris I.V. infusion 235mg launched in Japan
Jeselhy tablet 40mg launched in Japan
Contributing to Patients Worldwide With Innovative Japanese Medicine
Entering the 21st century, Taiho Pharmaceutical stepped up its global in-house development of new drugs that can contribute to cancer treatment. This led to the launch of LONSURF, an oral anticancer drug discovered in-house, in Japan in 2014. The following year, LONSURF received approval to be marketed in the U.S., becoming the first product the company itself sold in the U.S. market. In 2016, it was approved by the European Commission, and has been made available around the world. Additionally, leveraging its proprietary drug discovery platform technology, Cysteinomics, Taiho Pharmaceutical developed LYTGOBI which was approved in the United States (accelerated approval) in 2022, followed by approval in Japan and Europe (conditional marketing authorization) in 2023.
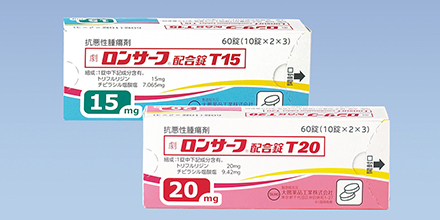
2014
LONSURF combination tablet T15/T20 launched in Japan
-
-
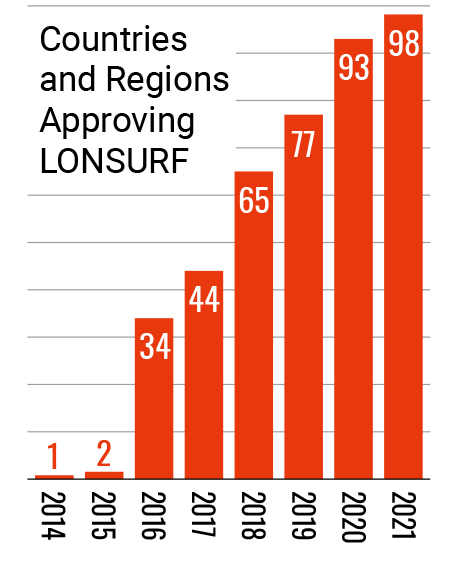
- (as of Dec 2020)
-
2022
LYTGOBI tablet 4mg launched in Japan